As of March 2023, 11 medical exoskeletons have been approved by the U.S. FDA for walking, gait assistance and physical rehabilitation. The FDA classifies medical powered exoskeletons as Class II devices, code PHL, with the exact definition as follows: "A powered exoskeleton is a prescription device that consists of an external powered, motorized orthosis placed over the paralyzed or debilitated abdomen of a person's extremity(s) for medical purposes."
The 11 medical exoskeletons are in no particular order:
- Keeogo Dermoskeleton System by B-Temia, powered knee exoskeleton.
- HAL for medical use (lower limb type) by CYBERDYNE, powered hip-knee exoskeleton
- EksoNR Ekso Bionics powered hip-knee exoskeleton
- ExoAtlet-II by ExoAtlet Asia, also a powered hip-knee exoskeleton.
- Honda Walking Assist Device, Honda Motor Company, powered hip exoskeleton.
- Indego, officially from Parker-Hannifin Corporation, recently acquired by EksoBionics, powered hip-knee exoskeleton.
- ReWalk from ReWalk Robotics, powered hip-knee exoskeleton
- ReWalk ReStore from ReWalk Robotics, motorized ankle exosuit
- GEMS-H from Samsung Electronics, powered hip exoskeleton.
- Phoenix by Ottobock, officially suitX (which itself was officially US Bionics), is a powered hip exoskeleton with passive knee support
- Atalante Wandercraft SAS powered hip-knee-ankle exoskeleton
As of March 2023, only the ReWalk exoskeleton is FDA approved for use with stairs and curbs. Only ReWalk Personal and Indego are approved for private or home use. All devices can be used in a rehabilitation center setting. The Atalante is the only self-balancing exoskeleton approved by the FDA (although the REX may soon be added to the list). The rest of the devices have either optional or required additional mobility aids such as crutches or a walker. There are several devices, such as the Atalante, that may be used by adolescents (but there are no pediatric exoskeletons on this list). All operators must complete a training program before using the exoskeletons.
Some of the FDA-approved powered exoskeletons have special fall protection and mitigation considerations, specifically:
- Atalante: must be used in combination with a safety railing.
- ReWalk must be used with crutches. The Indego, the ExoAtlet-II (and probably the Phoenix) should be used with crutches or a rollator, while the EksoNR can be used with crutches, a rollator or a walking stick.
- The HAL for medical purposes must be attached to a body weight support system (usually overhead).
- All versions of the ReWalk and Indego, with the exception of the home use versions, have been approved for use only under the supervision of a trained medical professional.
The intended Ese case for FDA-approved powered exoskeletons is to help patients/users walk with:
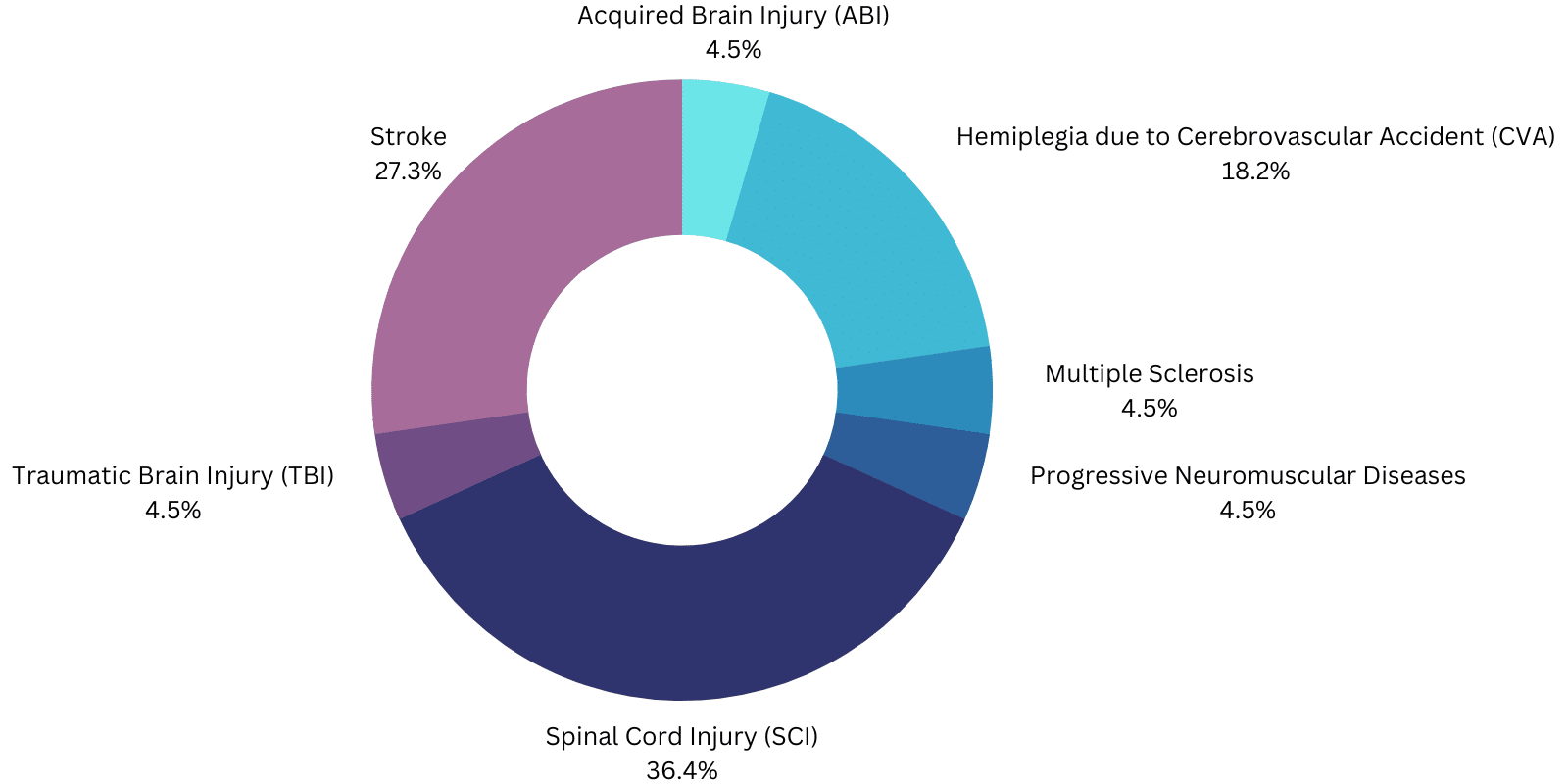
- ExoAtlet-II is designed for functional rehabilitation of individuals under the supervision of a trained physical therapist with upper extremity motor function of at least 4/5 in both arms, spinal cord injury (SCI) T4 to L5 or C7 to T3 (ASIA D ).
- Phoenix: spinal cord injury (SCI) of the T4-L5 level.
- Atalante: hemiplegia due to cerebrovascular accident (CVA).
- GEMS-H: Individuals with stroke who have gait deficits and gait speeds of at least 0.4 m/s and are able to walk at least 10 meters with the assistance of no more than one person.
- ReWalk: SCI T7 to L5 for personal use under constant supervision or SCI T4 to T6 for physical rehabilitation in a special facility.
- ReWalk Restore: should be "used to support ambulatory functions in rehabilitation facilities under the supervision of a trained therapist for people with hemiplegia/hemiparesis due to stroke who can walk at least 5 feet (1.5 m) with no more than minimal to moderate support ."
- Indego: Outpatient assistance for SCI levels T3 through L5 for personal use with monitoring and SCI levels C7 through L5 in rehabilitation facilities or hemiplegia due to CVA with motor function 4/5 in at least one upper extremity.
- Honda Walking Assist: "Individuals with stroke who have gait deficits and gait speeds of at least 0.4 m/s and are able to walk at least 10 meters with assistance from no more than one person" in rehabilitation facilities.
- EksoNR: "The EksoNRm shall perform outpatient functions in rehabilitation facilities under the supervision of a trained physical therapist for the following populations.
- Individuals with multiple sclerosis (upper extremity motor function of at least 4/5 in at least one arm).
- Individuals with acquired brain injury, including traumatic brain injury and stroke (upper extremity motor function of at least 4/5 in at least one arm).
- Individuals with T4 to L5 level spinal cord injuries (upper extremity motor function of at least 4/5 in both arms).
- Individuals with spinal cord injuries at levels C7 through T3 (ASIA D with upper extremity motor function of at least 4/5 in both arms).
Reported size and weight restrictions for users:
| Device: | Height range of the user | User weight range |
| GEMS-H | 1.55m to 1.91m | 45 till 100 kg |
| Atalanta | 1.60 to 1.90 cm | up to 90 kg |
| Phoenix | 1.60m to 1.87m | till 91 kg |
| ReWalk | 1.60m to 1.90m | up to 100 kg |
| ReWalk recovery | 1.42m to 1.92m | up to 120 kg |
| I need | 1.50m to 1.90m | up to 113 kg |
| Honda walker | 1.4m to 2.0m | up to 100 kg |
| ExoAthlete-II | 1.60m to 1.90m | up to 100 kg |
| ExoNR | 1.58m to 1.88m | up to 100 kg |
| HAL for medical purposes | 1.50m to 1.90m | 40 to 100 kilo |
| Keego's dermoskeleton system | 1.52m to 1.88m | up to 130 kg |
If you see any errors or would like an article on a specific topic, please do not hesitate to contact us via the ExR contact form . Featured image (above): Demonstration of the Ekso GT by Shane Mosko and physical therapist Jenn Macievich at Ekso Bionics headquarters, Richmond, 2016 (background removed).
Source:
- Product Classification, FDA, accessed March 2023, Link
- Palermo AE, Maher JL, Baunsgaard CB, Nash MS. Clinician-oriented review of the use of a bionic exoskeleton after spinal cord injury. Top Spinal Cord Inj Rehabil. Summer 2017;23(3):234-244. doi: 10.1310/sci2303-234. PMID: 29339899; PMC ID: PMC5562031.
- Evaluation of Safety and Performance of the Atalante System With Patients With Lower Limb
- Paralysis, NIH, 2018, Link
Originating source: proposed use of the 11 FDA-approved medical exoskeletons in the 2023 Medical Exoskeleton Report.